Abstract
HCT or CAR-T therapy recipients who develop COVID-19 have decreased overall survival (68% Sharma et al. Lancet Haematol. 2021), likely due to disease-inherent and therapy-related immunodeficiency. The development of specific COVID therapies and vaccination during the pandemic has changed the prevention and management of COVID-19 infection. Nevertheless, with the appearance of new SARS-CoV2 variants and omicron, in particular, questions about the severity of disease in high-risk patients are yet to be defined.
Methods
We included retrospectively 218 adult HCT/CAR-T recipients diagnosed with presumed COVID-19 omicron variant at Memorial Sloan Kettering Cancer Center (MSK), New York, between December 2021 and February 2022. Data were analyzed using SPSS version 26 (IBM, Armonk, NY).
Results
Among 218 patients, 107 received an autologous HCT, 92 allogeneic HCT, and 19 CAR-T. The median age was 58 years (range, 21 to 87), and 56% were male. The median time from HCT/CAR-T to SARS-CoV2 infection was 44 months (range, 0.1 to 272 months), and 27 patients (12%) acquired SARS-CoV2 within the first 100 days post-HCT/CAR-T. The primary hematologic diagnoses were plasma cell (37%), myeloid (37%), and lymphoid (26%) malignancies. Myeloablative conditioning was performed in 90 patients (41%). In the alloHCT recipients, 17 donors (18%) were matched related, 51 (55%) matched unrelated, 19 (21%) umbilical cord blood, and 5 (6%) haploidentical. 31% patients had 2 or more comorbidities and 9% received IVIG for hypogammaglobulinemia within the prior month. Of note, 40% of the patients were on active cancer therapy (maintenance or treatment) at the time of COVID-19 infection, and 47% of the alloHCT recipients were on GVHD immunosuppressive treatment.
Characteristics of COVID-19 omicron variant infection are shown in Table1. Specific COVID-19 treatment was administered in 111 patients (51%) and included MoAbs in 63 patients (56.7%), sotrovimab being the most frequently used (n=55, 87 %), remdesivir (n= 23, 21%), and other antivirals (n= 15, 14%).
Risk factors for admission were presence of > 3 comorbidities (HR, 6.2; 95% CI 2.3-16.3, p= 0.000), progression of disease (POD) (HR, 3.8; 95% CI 1.6-8.9, p= 0.002,) and CAR-T therapy (HR 4.5; 95% CI 1.4-14.1p= 0.008).
Regarding vaccination status, 84% (n= 183) of the cohort was vaccinated with mRNA Covid-19 vaccines, with 42% of patients having 1 booster before COVID19 infection. Interestingly, only 15/35 (42%) of the booster patients with measured serologic response showed SARS-CoV-19 Antibodies value> 2500 BAU/I.
Nineteen patients showed low titer COVID-19 antibodies (<2500 BAU/I) testing after COVID-19 infection: 3 patients died (2 for POD, 1 for COVID), 3 were not vaccinated, 1 patient had to be revaccinated after HCT, and 6 patients were administered tixagevimab/cilgavimab for future prophylaxis.
Persistent/Long COVID were present in 8 (3.5%) patients, showing fatigue and URI symptoms 5/8 (62%) had a previous admission for COVID-19 infection and 5/8 (62%) had a negative spike antibody titer before infection. The median duration of viral shedding (positive SARS-CoV-2 PCR) was 11 weeks (range, 3.2 to 19.1 weeks).
After a median follow up of 4 months (range, 0 to 8 months), 21 patients (9.6%) had recurrent COVID infection, and two of them died. The overall mortality rate was 10% in all patients (22/218 patients), with 2.5% (6/22) attributable to COVID. Other causes of death were progressive disease (n=13), sepsis (n=1), and hepatic VOD (n=1).
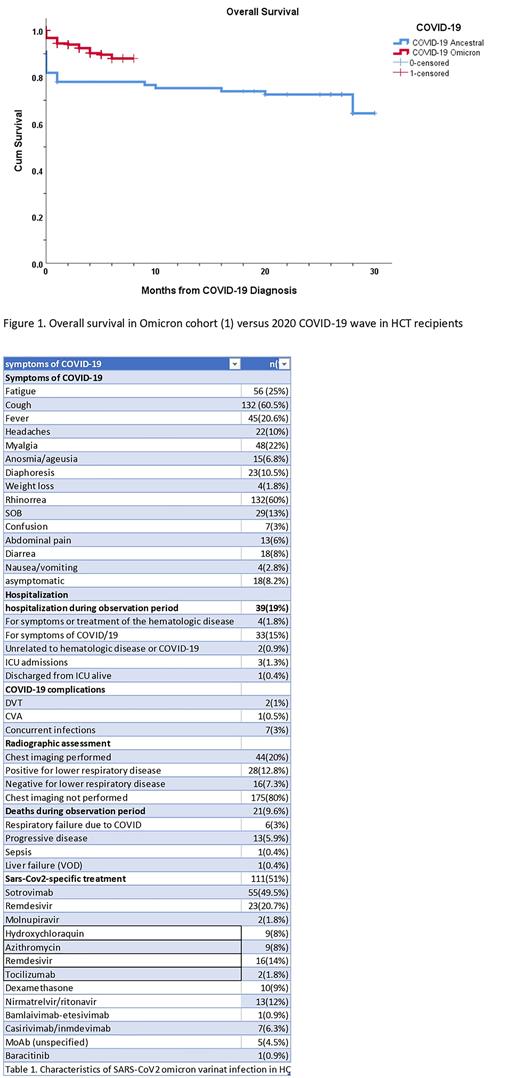
Comparing our omicron cohort with the initial 77 HCT & CAR-T patients infected with first wave COVID-19 in March 2020 at our institution, overall survival at 30 days was 94% vs 78% (p=0.010) (Figure 1).
Conclusions
In our cohort, mortality attributable to the COVID-19 omicron variant was low (2.5%), and only 19% required admission.
Risk factors for admission were presence of 3 or more comorbidities, POD at time of COVID infection, and CAR-T therapy.
Altogether our date confirms enhanced overall survival (94%) in HCT, and CART recipients infected with the omicron variant of SARS-CoV2 as compared to prior waves of the COVID-19 pandemic.
Access to SARS-COV-2 specific treatments and the higher incidence of vaccination in this cohort appear to be key factors to successfully managing patients in order to reduce COVID-19 mortality. Pre-exposure prophylaxis became available later and is also likely important.
Disclosures
Politikos:Merck: Research Funding; PrecicionHeor: Honoraria; ExcelThera: Membership on an entity's Board of Directors or advisory committees. Barker:Merck: Research Funding; New York Blood Center: Consultancy; Gamida Cell: Consultancy. Perales:Servier: Consultancy; Medigene: Consultancy; Karyopharm: Honoraria; Celgene: Honoraria; MorphoSys: Consultancy, Honoraria; Takeda: Honoraria; VectivBio AG: Honoraria; Vor Biopharma: Honoraria; Cidara Therapeutics: Consultancy; Orca Bio: Consultancy; Omeros: Consultancy; Abbvie: Honoraria; Astellas: Honoraria; Sellas Life Sciences: Consultancy; Bellicum: Honoraria; DSMB: Other; Nektar Therapeutics: Consultancy, Honoraria; Novartis: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Merck: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria. Shah:Amgen: Research Funding; Beyond Spring: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal